Max Nieuwdorp – We Are Our Hormones
(pp. 13-22)
Introduction
In 2001, I worked in a rural hospital in Pretoria, South Africa. Pregnant women from the townships would come to the rudimentary maternity hospital with starting contractions. Lying on a flattened cardboard box on the grass outside the building, they would handle their contractions until the moment they swapped the cardboard for a hard bed behind a curtain inside and the delivery could begin. On average, I had about twenty women under my care. Several children would be born every night and I would spend my time running from room to room. One of the children, a girl called Muna, came to see me in the outpatient clinic a while later due to stunted growth. She barely responded to attempts to communicate with her, had a puffy face and delayed reflexes. The thyroid hormone in her blood was immeasurably low, so I decided to administer her thyroid hormone tablets immediately to address the deficit.
When I visited the same maternity hospital years later while I was in South Africa for a conference, a nurse told me that Muna was severely disabled and cared for at home by her grandma. Muna’s first few months without any treatment had taken their toll. She would never be able to live independently and had an increased risk of dying prematurely due to pneumonia or bedsores.
Muna’s story shows how important hormones are for our development. We simply cannot do without these substances that your body makes itself and that steer organs and tissues, via the bloodstream, to regulate all sorts of bodily functions. At first, an unborn child is dependent on its mother’s hormones. Only after three months does a foetus develop the cells and organs needed to effectively produce hormones itself. The thyroid is formed in the first trimester of the pregnancy, which illustrates just how crucial this organ is for our existence; the thyroid hormone is involved in many of our bodily processes.
Due to a disturbance in that first trimester Muna’s thyroid gland failed to develop and she ended up with a congenital deficit of thyroid hormone, also known as congenital hypothyroidism (CH). In the Netherlands, approximately eighty children each year are born with this condition. It is not easy to make this diagnosis in newborn babies and if it is only made relatively late, as was the case with Muna, it can have major consequences. This insight led Hans Galjaard, Emeritus Professor of Human Genetics at Erasmus University Rotterdam, to put screening for congenital conditions in the Netherlands on the political agenda. Thanks to his efforts, Dutch hospitals and maternity units have been taking blood samples from all babies since 1974. Partly motivated by the fact that his brother died of a congenital disease at a young age1, Galjaard initiated these heel-prick tests, which now screen for thirty-two congenital diseases, after an exhaustive political lobby. In Galjaard’s words: ‘Better to prevent than not to be able to cure.’
As a result, thousands of children have been spared the fate of Muna. I see them in my clinic as lively thirty-somethings, whose lives have been changed forever thanks to that one thyroid tablet per day (and Galjaard’s forward-looking view).
A brief history of hormones
The substance hormone was first referred to as such by the British physiologists Ernest Starling and his brother-in-law William Bayliss in 1902. They studied how our digestive system works and how food can be broken down and absorbed by certain compounds in the gut.2,3
Two years later, Ivan Pavlov won the Nobel Prize for Physiology or Medicine for his research into the digestive system.4 This Russian colleague, chiefly known for his research into conditioning and after whom the infamous Pavlov reaction is named, used his experiments to demonstrate how the nervous system is involved in our digestion.
However, Bayliss and Starling established that digestion also took place in laboratory animals with damaged nervous systems, by releasing special substances into the blood from nearby glands. One of these substances was what they called secretin (from ‘to secrete’) — the first of a rich group of substances that control our lives in an invisible yet far-reaching way.
It was also Bayliss and Starling who proposed the term hormone — Ancient Greek for ‘impetus’ or ‘to set in motion’ — as a collective name for these substances. Hormones are signalling molecules created by endocrine (hormone-producing) glands. These molecules travel via the blood and other bodily fluids to their destination — specific cells or organs — where they do their work. Most hormones have a central control function; they can set processes in motion or slow them down. They also interact with each other.
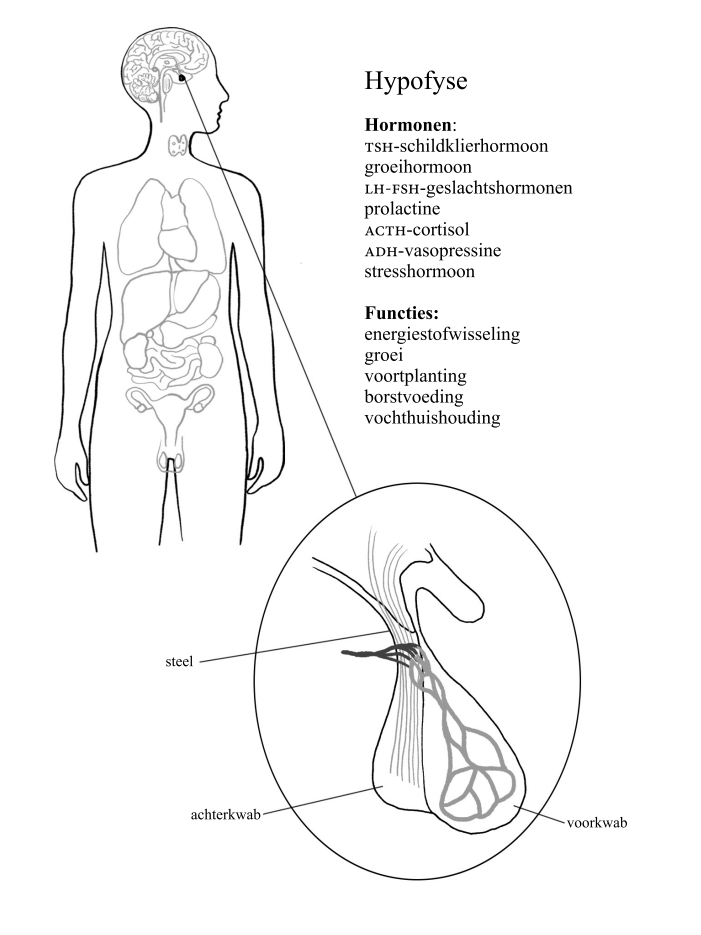
The headquarters of our hormonal housekeeping is in the centre of the brain, right behind our eye sockets. That’s where the hypothalamus and the pituitary gland are located, the size of a strawberry and a pea respectively. Both groups of specialised nerve cells form part of our emotional brain, the limbic system (read more about this in chapter 5). They lead both our nervous system and our endocrine system like army generals, keeping a close eye on all the troops.
The effects of these important signalling molecules had, however, already been observed fifty years prior to Starling and Bayliss. In an experiment from 1849, the German scientist Arnold Berthold compared castrated male chickens (capons) with their non-castrated brothers, and found that the first group experienced physical and behavioural changes.5 What was striking was that when the testes were restored (by re-implantation or transplantation), and with it the production of the later-discovered hormone testosterone reinstated, the chickens were able to crow again. Similar experiments continue to capture the imagination of writers and scientists to this day, not least because they allude to the existence of an elixir for ‘eternal’ youth.
The opera A Dog’s Heart by composer Alexander Raskatov is a wonderful example of this. Inspired by a novella by Mikhail Bulgakov from 19256, the opera tells the fate of the street dog Sharik after being implanted with the pituitary gland and testicles of a notorious criminal. The animal turns into the unscrupulous delinquent Sharikov, whose behaviour and choices fall prey to his (hormone-controlled) urges. Only a second operation is able to offer salvation to this testosterone-riddled dog…
Older literature such as the Old Testament also refers to the presence of hormones. Although techniques to demonstrate the presence of hormones in the blood did not exist at that time, their ‘impetus’ is described: ‘the life of flesh is in the blood’ (Leviticus 17:11). It is highly likely that certain characters in the Bible had congenital hormonal conditions, such as the giant Goliath, who likely suffered from excessive growth hormone. The dwarfism of the Egyptian god Bes and the irritability and enormous energy of Cleopatra could also have been caused by an abnormal thyroid gland.
Back to the fascination with the male hormone for eternal youth. In 1889, the 72-year-old Mauritian-French neurologist Charles Brown-Séquard experimented by injecting himself with testicular extracts from animals.7 ‘In my self-administered subcutaneous injections I used a watery fluid with the following three extracts: first, blood of the testicular veins; secondly, semen; and thirdly, juice extracted from a testicle, crushed immediately after it had been taken from a dog or guinea pig.’ Although the professor was relatively healthy for his age, in the period before his self-experiments he had complained regularly of fatigue after a hard day’s work and reported suffering from heartburn and painful joints and muscles. The latter was likely wear and tear as a result of osteoarthritis, which is very common among older people.
In May and June of that year, Brown-Séquard injected himself as many as ten (!) times every day. Almost immediately, the vitality and energy seemed to return to his body; he felt stronger and could literally run upstairs again. The circumference of his biceps also appeared to significantly increase, he was no longer fatigued and, according to legend, he got his potency back. Testosterone (more about this in the following chapters) is, however, a fat-soluble hormone and given the fact that Brown-Séquard’s injections were water-based, it is perfectly possible that a placebo effect was at play here.8
This and other examples have had a whirlwind effect on our knowledge about hormones over the past century. Thanks to technological progress, hormones can be isolated from animal material and subsequently injected in order to observe their effect on humans and other animals. This has not only led to many important, new insights for medical science — resulting in multiple Nobel Prizes between 1920 and 1930 for the discovery of today’s best-known hormones oestrogen (female hormone), testosterone (male hormone) and progesterone (which plays an important role in the implantation of the embryo in the endometrium) — but it has also had major social, societal and economic effects. The development of ‘the pill’ in the 1950s, for example, had a tremendous impact on the emancipation and empowerment of millions of young women. Furthermore, the general burden of disease decreased significantly thanks to successful treatments using hormones for numerous conditions, which simultaneously led to huge opportunities for the pharmaceutical industry.
Unfortunately, our hormonal helpers haven’t always made a good impression. Since the publication of Rachel Carson’s Silent Spring in 1962 — in which the American biologist referred to the disastrous influence of agricultural pesticides on the environment, the quality of our food and our own bodies — we have a better understanding of the extent to which these toxins are able to derail our hormonal balance.9 Injections with growth hormone taken from brain glands of human corpses resulted in many patients contracting the infectious and fatal Creutzfeldt–Jakob disease (better known as mad cow disease).10 The medicine DES, an artificial oestrogen widely prescribed to pregnant women in the Netherlands in the 1950s and 1960s to prevent miscarriages, also proved to have major consequences for the health of their daughters, such as an increased risk of cancer and infertility; it was found to even potentially lead to abnormalities in their grandsons.11
As was the case with Muna, the baby who ended up with an intellectual and physical disability as a result of defective thyroid hormone production, our health and that of our offspring is highly dependent on the right hormonal balance. In this book, I will explain the influence of the various hormones and their interrelationship throughout the various life phases (from the cradle to the grave). I will also delve deeper into the consequences of a lack or excess of hormones and the (sometimes destructive) effect of these powerful endogenous substances on our mental and physical well-being. I hope, like me, you will be fascinated by the amazing role that hormones play in our bodies and our lives.
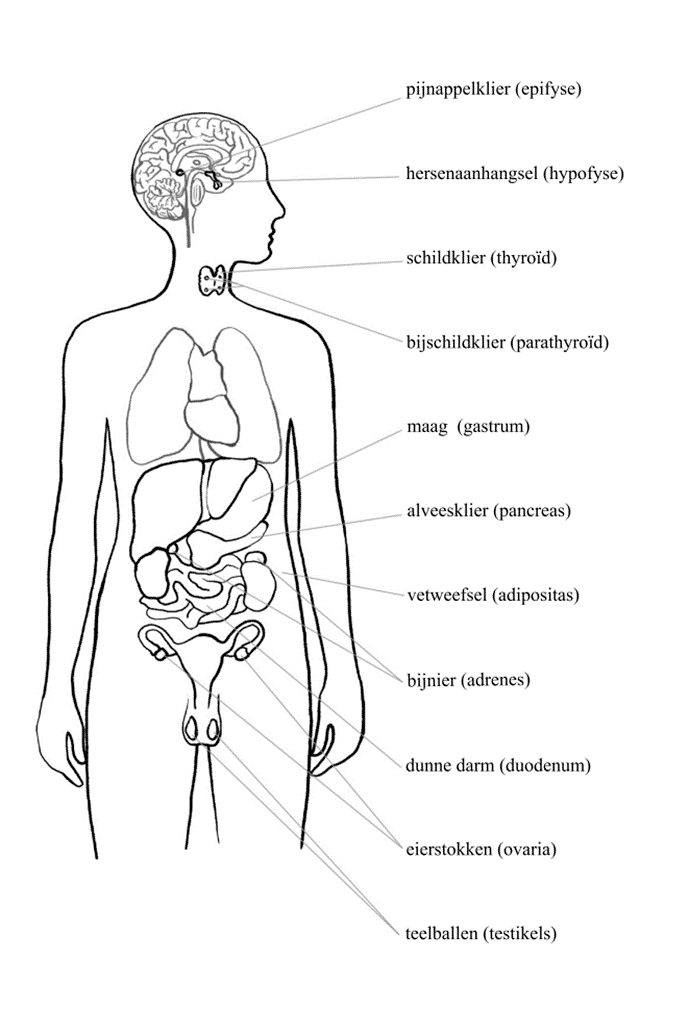
Our endocrine glands and their functions
Pituitary gland (hypophysis), conductor of our body; quantity: 1, size 1 × 1 cm; looks like: a pea. Produces growth hormone, prolactin, luteinising hormone (LH), follicle-stimulating hormone (FSH), adrenocorticotropic hormone (ACTH) and antidiuretic hormone (ADH), also known as vasopressin. Function: puts other glands to work to produce hormones.
Pineal gland (epiphysis); quantity: 1, size 0.5 × 0.5 cm; looks like: a pineapple. Produces melatonin. Function: circadian rhythm and sleep quality, impedes the production of sex hormones until puberty.
Thyroid gland; quantity: 2, size 5 × 3 cm; looks like: the wings of a butterfly. Produces T4 and T3 (via TRH and TSH from the pituitary); controls metabolism, heart rhythm and body temperature.
Parathyroid gland; quantity: 4, size 0.5 × 0.5 cm; looks like: a grain of rice. Produces parathyroid hormone (PTH), important for bone quality and calcium regulation.
Stomach; quantity: 1, size 30 × 10 cm; looks like: an inverted pear. Produces ghrelin (hunger hormone) and gastrin. Function: digestion.
Pancreas; quantity: 1, size 14 × 3 cm; looks like: a flat pear. Produces insulin and glucagon. Function: sugar level and fat metabolism.
Fat tissue; throughout the body, especially abdominal area, size varies; looks like: semolina pudding. Produces leptin and oestradiol (from testosterone). Function: energy supply, elasticity of the skin.
Adrenal gland; quantity: 1, size approx. 1 × 1 cm; looks like: a thimble. Produces aldosterone, cortisol, oestrogen, DHEA (dehydroepiandrosterone) and testosterone under the influence of CRH (corticotropin-releasing hormone) from the hypothalamus and ACTH from the pituitary. Important for: blood pressure, maintaining sugar and salt levels, the immune system and the libido. The adrenal medulla produces (nor)adrenaline, important for the stress response.
Small intestine (duodenum); quantity: 1, size 120 cm (twelve fingers); looks like: a bicycle tyre. Produces cholecystokinin (CKK), serotonin, glucagon-like peptide (GLP-1). Function: digestion.
Ovaries; quantity: 2, size 5 × 3 cm; look like: almonds. Produce oestrogen, progesterone and testosterone under the influence of GnRH (gonadotropin-releasing hormone), FSH and LH via the hypophysis. Function: menstrual cycle, breast development, reproduction, bone mass and quality.
Testes (testicles); quantity: 2, size 4 to 5 cm; look like: little Easter eggs in a pouch. Produce testosterone. Important for sperm, reproduction, sexual desire, muscle mass, beard growth, bone mass and quality.
(pp. 57-59)
2. The big run-up
Pubescent toddlers
I wake up on New Year’s Eve 2010 in high spirits. It’s been a good year, I’m happy with my wife, our third child is on the way and I’ve just started training to become an endocrinologist in Amsterdam. Life is treating us well, so it seems. I go over to our two-year-old son, who’s lying in bed as pale as a sheet and gently calling out ‘ow, tummy, ow’. Over the past few months, he hasn’t been eating as well as usual and he’s woken up a few times at night soaked in sweat, but that hadn’t given us cause for concern.
When I lift him out of bed, I feel a hard lump just under his right ribcage. At first I think I’m imagining it — I’ve never seen or felt that lump before — but eight hours later our life has been turned upside down. The diagnosis: childhood liver cancer with metastases. It is unlikely he will make it through the next year.
In the following days, a line from Franz Schubert’s Erlkönig haunts my thoughts like a cadence. The musical work is based on Goethe’s poem from 1782, in which a father carries his ailing child to a doctor at night by horseback. Meanwhile, the son, whose condition is rapidly deteriorating, deliriously mumbles that the Erlkönig is taking him away to the underworld: ‘My father, my father, he has seized me! Erlkönig is harming me!’
The months that follow are incredibly stressful for our whole family. We stay at Amsterdam University Medical Centre day and night and sleep badly without exception. Our son has to undergo a liver transplant with part of my own liver; that means a serious operation for the pair of us in Brussels. Stress hormones surge through our bodies.
Fortunately, things worked out well for our son in the end, but our youngest daughter was exposed to elevated levels of the stress hormone cortisol in the womb for almost five months. Although we don’t know the long-term effects of this, we had many a sleepless night in the
first three years of her life because she turned out to be an irritable greedy guts. Although she’s doing well now too, she has to pay special attention to what she eats because she gains weight much more quickly than the rest of our family.
This can probably be attributed to a mechanism called ‘epigenetic change’; if you are exposed to the stress hormone cortisol early in life, certain genes are differently tuned for the rest of your life.1 Some of our hormones have this powerful effect; your genes themselves don’t change, but they function less effectively, which is detrimental to your health.
The effects of hormonal fluctuations during a pregnancy can have lasting effects on the child’s developing brain and body.2 Severe chronic stress during pregnancy (such as natural disasters or wars) can cause irreversible damage to the neurological development of foetuses — just think, for example, of the children born during the Second World War in the Dutch famine of 1944. In such cases, the child’s critical development period coincides with the period in which the stress hormones in the mother’s blood are elevated.
Since these hormones affect our immune systems and our brains, the effects are reflected in numerous physical and mental behaviours. This can manifest itself later in life in all sorts of ways, such as poor concentration, fear (of failure), deficient general functioning and possibly even schizophrenia. In a large-scale Danish population study among 1.3 million babies born between 1973 and 1995, researchers found a link between major stress and intense grief in the first trimester of the pregnancy (such as the death of a loved one or another trauma) and an increase in the number of deformities and premature births.3
Another study revealed that emotional stress in healthy pregnant women is also detrimental to the motor and cognitive development of their two-year-old children.4 Unfortunately we are unable to predict for whom early hormonal fluctuations in the womb will lead to problems. This is an extremely complex matter, but in general the earlier and more intense such a cocktail of signals presents itself, the greater the impact will be. Hormones can therefore influence our futures a bit like something out of a science-fiction film.
In this chapter you will read more about the far-reaching influence of hormones on young children, primarily in terms of the effects of the sex hormones on toddlers’ bodies. You will see that these young children encounter a storm of hormones in the first four years of their lives and what this can mean both physically (appearance, fertility) as well as psychosocially (behaviour). I also explain what can happen if you are exposed to too many hormones at an early age — whether from your own body, or from the outside world via food, toys or pesticides.
(pp. 163-165)
6. Key players in your hormonal balance
The residents of your gut
In autumn 2006, I was working as a junior doctor at Amsterdam University Medical Centre. A melancholy patient in her eighties had been there for many months; she had developed chronic diarrhoea from a course of antibiotics for a urine infection and no medication could help her anymore. The diarrhoea was the result of an infection caused by Clostridium difficile; this bacterium damages the intestinal wall and leads to severe abdominal pain, fever and cramps. Patients get bouts of watery diarrhoea up to ten times a day, causing them to lose weight and end up so weak they are completely bedridden. Twenty percent of patients with this infection end up succumbing to it. It’s hardly surprising that many of them feel melancholy. As the woman had been in bed with depression for so long, she was completely emaciated and had bedsores all over her body. It was a very serious situation.
The woman wanted nothing more than to leave the hospital to be at home with her husband (who had metastatic cancer). I understood that only too well and so off I went, perhaps overconfidently, in search of a treatment that would provide a short-term cure. Without success, until I remembered a hilarious presentation at a conference a few years earlier, where the Norwegian professor of gastroenterology, Johannes Aas, had explained that he had been known to treat these types of cases with the poo of a healthy person, since reading a publication about it from 1958.1 By infusing healthy stool with other bacteria, and so without the Clostridium-difficile bacterium, the proliferation of this bothersome bacterium is impeded.2 As a result, there would be a battle between the bacteria, whereby the healthy type — which is best at multiplying — wins by ousting the bad Clostridium bacterium from the gut. What happens daily in nature can be given a helping hand inside a body by means of a faecal transplantation. Only years after the talk by the Norwegian doctor did the importance of his words dawn on me.
Within a week — after gaining the patient’s consent — I had everything I needed for the first faecal transplantation in the Netherlands. I would have never thought you could use a simple kitchen centrifuge for the preparation of a stool solution… One Friday afternoon we treated my patient by inserting her son’s stool via colonoscopy. I could sense the excitement in the treatment room as everyone present, from nurse to doctor, felt that something very special was about to happen.
The patient was safely returned to her room in the hospital that afternoon and, at first, nothing struck us. However, when I returned to the room after the weekend, it appeared that her chronic diarrhoea had, in fact, disappeared. The woman had been for a little walk for the first time in months and was in a great mood. She didn’t have any side effects of the treatment, except that her melancholia had left without trace. The following day she went back home, together with her husband. They enjoyed life together for a number of years.
The bacteria in your gut are indispensable for a healthy hormonal balance. They are involved in the release and production of dozens of different hormones.4 Gut bacteria influence the creation of hormones and the function of the brain via the central nervous system.5 Furthermore, bacteria in general are often the source of the discovery of new medicines, ranging from antibiotics to cholesterol-reducing medication.6 In summary, the bacteria in and around us are extremely important for a healthy life!
(pp. 170-174)
The residents of our guts
In 2002, I did a placement in the ear, nose and throat department at the Charité hospital in Berlin. I was shadowing a professor who had trained in East Germany, who told me the first time I met her: ‘A good trainee doctor doesn’t ask questions, but listens.’ So, while I sat beside her on a chair in silence, I became familiar with all the infections that present themselves in the head and throat area.
That’s where I fell under the spell of Robert Koch (1843-1910) and his research into the influence of bacteria on the development of infections. Koch was a rural GP in Wolsztyn in Poland (Prussia at the time) and became fascinated by the saliva, stool and wound exudate of his patients — especially after his wife gave him a microscope as a birthday present. In a small corner of his GP practice he worked on one of the biggest discoveries in the world of medicine, rightfully awarded the Nobel Prize in 1905. What did he discover? That bacteria can cause infections.
After Koch started work in the Charité hospital as a professor (and walked the same corridors that I would walk almost a hundred years later), he published ‘Koch’s postulates’.17 These are a number of rules of thumb that doctors and scientists can use to establish whether certain bacteria and viruses cause human diseases. According to his criteria — just like we saw in the COVID-19 pandemic — a bacterium or virus (or pathogen) must firstly be encountered in large numbers in a sick person. Secondly, it must then be possible for the pathogen to be isolated and cultured. Thirdly, after exposing a laboratory animal to the pathogen, it must cause the assumed disease (and later cure it again during treatment). Finally, the bacterium from the experiment with the animal must be the same as the one encountered with the patient.
This leads us to the question: how can we transfer bacteria from one human to another in order to strengthen the effect of our hormones?
After successfully carrying out the first Dutch faecal transplantation in Amsterdam in 2006, the treatment was applied equally successfully to a similar group of patients in a large-scale study.18 Since then, it has been included in the arsenal of treatments for chronic Clostridium difficile diarrhoea all over the world. As an endocrinologist I became more and more interested in the effects of donor faecal transplantations on the hormonal balance. We discovered, for example — this was later confirmed by other research — that a change to the gut bacteria composition makes a body more sensitive to insulin19 and that the effect of serotonin and dopamine decreases in the brains of overweight people.20 Think, for example, of the lack of serotonin, which immediately makes people feel down. Sterile mice that have never had a bacterium in their guts are more anxious than mice found in the wild, and are less able to learn new things. Their digestive tract is also less well-developed.21 To me, this seemed like a good reason to take a closer look at which friendly residents our guts are hosting and which ‘squatters’ we ought to be aware of.
Generally speaking, bacteria group together in complex communities — referred to by the American microbiologist Joshua Lederberg as ‘microbiota’. In our bodies, most of these are found in the gut. Until fifteen years ago, it was only possible to see which bacteria were on the skin or in the stool by taking a swab. The organ, stool or liquid of which the bacterial composition was being analysed would be swabbed, before being rubbed around a ‘nutritious’ plate (containing sheep blood) a few times to reveal which bacteria were growing.
A difficult undertaking, because if there is not quite enough bacteria on the swab or if the bacterium is unable to withstand light, for example, nothing grows. It is easy to see how this broad method gave rise to a huge underestimation of the number of bacteria in the gut. That’s why at that time, people assumed that only approximately three hundred types of bacteria lived in the gut. Since then, major progress has been made in terms of being able to establish the exact composition of our gut. This is possible thanks to high-throughput techniques that allow us to analyse bacterial DNA. It is not necessary to explain exactly how it works here, but this state-of-the-art technology has allowed us to establish that in a healthy gut, there are thousands of different types of bacteria per gram of stool. That’s well above those three hundred — and that’s before taking viruses and fungi into account. What’s more, these gut residents also release chemicals from our food. It was recently established, for example, that each day a quantity of alcohol is produced in our guts that is equivalent to three bottles of beer in a healthy person and up to half a litre of whisky in obese people.22 There’s a small brewery in your tummy; who would have guessed!
So there are many types of bacteria, with far-reaching impact. The magic box that the population of bacteria in our gut actually is, appears to be infinitely more than the once-held idea of a simple digestive tract. You can think of the gut microbiome (with fungi and viruses in addition to bacteria) as an organ weighing roughly two kilograms that fulfils many different functions. As mentioned, it produces favourable hormones such as serotonin and dopamine, important for our mood, but also supplies harmful substances, such as the hormone-like substance kynurenine, made from tryptophan.23 An excess of this is found in the blood of patients with inflammatory bowel diseases and can cause low mood and fatty liver. But we also recently discovered that the immune cells from our bone marrow firstly go to the small intestine, where they learn to recognise good and bad bacteria — a type of primary school for our immune system— before fulfilling their role as defenders of our body.24 This education sometimes goes wrong, causing the immune cells to turn against our hormone factory, resulting in an exhausted thyroid gland.25 I will look into this in more detail later in the book.
In summary, your gut bacteria are vitally important. In addition to taking care of the immune system, they derive as much energy as possible from your food.26 At least, that is, if it stays in your gut long enough. Overweight people have slower bowels (the transit time); this is due to worse gut bacteria composition.27 For mice, that even affects body temperature and therefore also metabolism.28 Since a diverse microbiome contributes to a better quality of sleep,29 jetlag can shake up our internal residents in such a way that it disrupts the release of the sleep hormone melatonin.30 It is logical that your metabolism is then affected too.
But what about the squatters, those bad bacteria you would rather not be housing? Don’t forget that you have a built-in alarm, your immune system, which can keep intruding pathogens out. Your own gut bacteria also work together to successfully ward off pathogens. When, for example, a bacterium that is resistant to antibiotics threatens to get the upper hand, the healthy intestinal flora respond immediately by providing extra protection to the intestinal lining.31 The new resident is ‘taken hostage’ and a major squatter movement cannot be formed.
In addition, gut bacteria work together by producing natural toxins to inhibit the growth of other bacteria. The best-known example of such a toxin is penicillin, which like almost all other antibiotics provides one bacterium with a weapon to eliminate the other bacteria. Bacteria can also make bacteriocines, which destroy the bad bacteria in our guts,32 such as those that are able to sustain a chronic stress reaction in our body.33 In this way, the human body and gut bacteria have a mutually beneficial relationship.
(pp. 269-274)
10. Old and cast aside
The beginning of the end
Once a year, Ludo comes to my outpatient clinic. At the age of 94, the widower is still full of life and spends half the year on one of the Spanish costas. Although he shuffles into my room with a slightly hunched back, it strikes me that he always seems younger and sprightlier than his age would suggest. He often makes a remark about how he likes to flirt with the ladies and that he hasn’t ‘lost his touch.’ With his sumptuous grey hair, Ludo still looks strikingly good and, above all, perky; I’ve never once heard him complain. He has a slim, wiry build and despite an arthritic hip, shoulders and knees, he gets up out of his chair with relative ease.
As he leaves, he cheerfully announces that the array of age-related diseases — an underactive thyroid, prediabetes and high blood pressure — won’t get the better of him and that ‘the glass is still half full.’ Even after his recent cataract operation, which allowed him to see ‘like an eagle’ again, he managed to continue living independently with just a bit of assistance. He doesn’t eat a lot and normally sticks to fish, vegetables and a small glass of wine. Ludo doesn’t feel old (‘Doctor, I feel about 50’) and as a retired PE teacher he is used to plenty of exercise; he kept that up after retirement. In Spain, he swims in the sea every day and walks along the beach to the neighbouring village for the shopping. The ten-kilometre round trip, which he would have once been able to run in under an hour, takes him four to five hours. His optimistic outlook and his constitution (which he has managed to maintain his whole life through lots of exercise) make Ludo a healthy old man.
In this final chapter I will describe the role of hormones on mental and physical health during the last phase of life in elderly people. And what we can do (sometimes even decades sooner) to grow old in the healthiest possible way.
What happens, in terms of hormones, from the age of 80 onwards? Three hormonal changes take place in the oldest old people: they lose contact with their day/night rhythm as a result of worsening sleep quality, which disrupts the production of hormones; the quality of their bones and muscles continues to decrease; and their sense of smell deteriorates. As people aged 80 and above experience loss of appetite and therefore eat less, they are less fit in general. These factors reinforce each other; a reduced appetite and lower intake of food in turn lead to worse sleep and fewer hormones being released.
The first person to refer to the disruption of this hormonal balance in elderly people, prior to the Second World War, was the American physiologist Walter Cannon.1 He developed the theory of homeostasis, building on the findings of his French colleague Claude Bernard. Cannon determined that a person’s physiological reserves that are capable of restoring their physical balance decrease as that person ages. This results in worse outcomes if they fall ill. I often tell junior doctors that with elderly patients, once the house of cards starts to wobble, a bad outcome is often inevitable.
That’s not any different for their hormonal housekeeping. As already mentioned, the 24-hour rhythm of our biological clock (also known as the circadian rhythm – circa means ‘about’, dies means ‘day’) disappears when our sleeping pattern changes, which also disrupts the release of hormones. The concentrations of hormones during the day have less pronounced peaks and troughs (lower pulsatility), similar to low and high tide. The elderly body responds to this by continually producing more hormones such as insulin or leptin, resulting in a type of resistance.2 It is precisely the fluctuations in high and low concentrations that are important for a well-functioning body; this enables it to respond flexibly during periods of illness. This loss of hormonal flexibility, as a result of which slightly elevated cortisol levels can be observed, appears to be a reliable predictor of dementia and premature death among elderly people.3,4
Another important hormonal change is that the sex hormones (notably testosterone) further decrease at this age, causing the muscle quality to decline dramatically.5 As the body also produces less vitamin D and therefore less calcium is absorbed by the intestines, the production of the parathyroid hormone (PTH) has to increase if sufficient calcium is to be released from bones for adequate blood levels, resulting in osteoporosis. In addition to this, changing (sex) hormones cause the optic nerve and the ear to work less effectively, causing our sight and hearing to deteriorate. 6,7 The consequences can be disastrous for old people; as they are more prone to falling, they break bones more often, which don’t heal as well either.
Eternal life
Although we now know our hormones can do many things, one feature in particular attracts a lot of interest: the possibility to prolong life. And perhaps even to let us live eternally.8 In 1939, the English scientist Aldous Huxley (yes, of Brave New World) wrote After Many a Summer9, in which he ridicules the temptation to stay forever young in California (to where he had just moved at the time). The main character, Jo Stoyte, is a millionaire who is scared to death of dying. He decides to hire Dr. Obispo to see what is scientifically possible. Obispo (inspired by the diaries of the Fifth Earl of Gonister, who had frantically attempted to find the secret to eternal life two hundred years earlier) suggests that the use of carp guts and their contents (i.e. a type of faecal transplantation) could be beneficial. At the end of the book, Stoyte and Obispo visit the Fifth Earl of Gonister, who is still alive, but now lives in a dungeon and resembles an ape. Jo Stoyte decides not to undergo this treatment after all.
What is interesting is that a few years before Huxley finished his novel, he himself studied carp to find out how these animals can live for so long (more than a hundred years, he believed). In his book he describes a treatment using the transplantation of carp faeces to transfer certain hormones from fish to human.
Nowadays, the British gerontologist and bioinformatician Aubrey de Grey is the oft-quoted apostle of the ‘immortality gospel’ based on (growth) hormones. His interest in human immortality (and what we can learn about it from other animal species) is certainly nothing new — in fact, it dates back hundreds of years. De Grey believes that we, just like in the Old Testament, can live to a thousand as long as we ensure our organs are frequently serviced.10 This would involve the body’s cells being regularly replaced with stem cells. This is what his research has focused on for the past two decades. And one of the substances he puts his proverbial money on is the hormone melatonin.
One of the first accounts of the quest for eternal life dates back to around two and a half centuries before Christ. After watching the suffering and death of his best friend Enkidu, Gilgamesh, demigod and king of South Mesopotamia,11 decided to make the discovery of a rejuvenating elixir his life goal. Fearing the fate of his friend, he visited many wise men to find out how he could prevent his own demise. One of his advisors was Utnapishtim — literally, ‘he who saw life’ — the heroic survivor of the oldest flood myth (similar to that of Noah) and himself immortal due to his relationship with the goddess of Life.12 Utnapishtim told Gilgamesh that eternal life could be found at the bottom of the ocean. Gilgamesh’s personal quest failed, but his example has been widely followed.
Around thirty years ago, a German biology student made a chance discovery while researching plankton in seawater off the North-Italian coast.13 Miniscule jellyfish were found in the water samples, but once they entered the laboratory these animals appeared to change form. Slowly but surely, they reverted back to their primary state, like butterflies becoming caterpillars again. The animal in question was the Turritopsis dohrnii: the ‘immortal jellyfish’. This is still the only species of animal that, after successfully developing into an ‘adult’, can revert to its original state, and subsequently resurrect itself as a healthy creature.14 This process takes place in response to physical or environmental stress. One of the internal triggers for the reverse life cycle of the jellyfish is reaching a certain age. Three guesses for which actors hold sway here: hormones! As, believe it or not, the juvenile hormones that regulate this process in jellyfish appear to be controlled by substances akin to our growth hormone.15
Translated by Alice Tetley-Paul